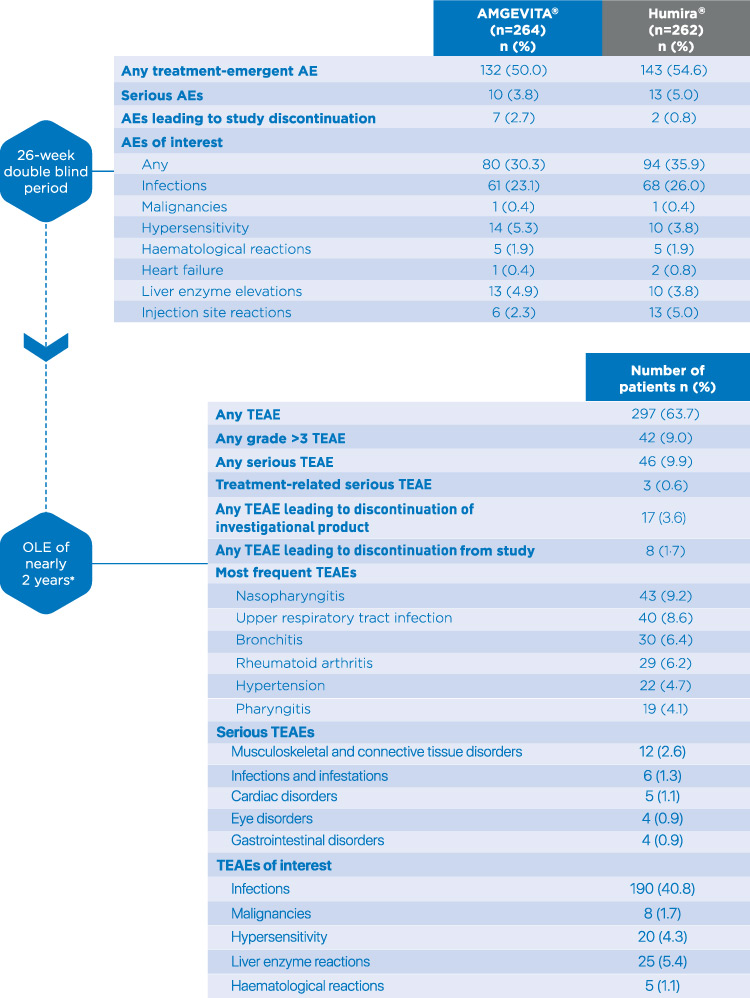
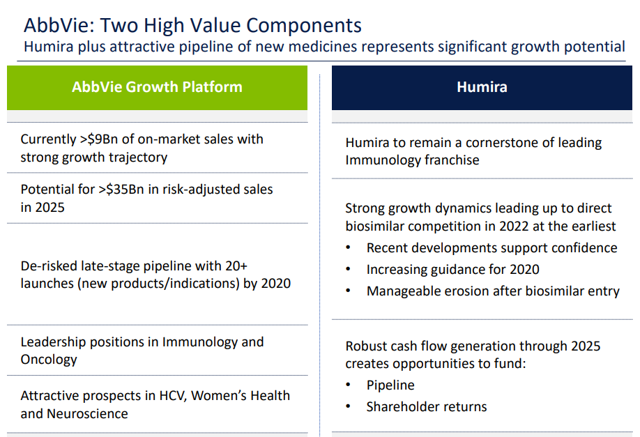
Overview of Humira® Biosimilars: Current European Landscape and Future Implications - Journal of Pharmaceutical Sciences

Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open- label extension study - ScienceDirect

Humira 40 mg/0.4 ml Pre-filled Syringe and Pre-filled Pen (Great Britain) - Summary of Product Characteristics (SmPC) - (emc)

These highlights do not include all the information needed to use IMRALDI safely and effectively. See full prescribing information for IMRALDI.IMRALDI (adalimumab-xxxx) injection, for subcutaneous useInitial U.S. Approval: YYYY IMRALDI (adalimumab-xxxx) is