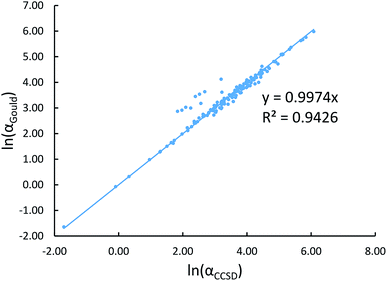
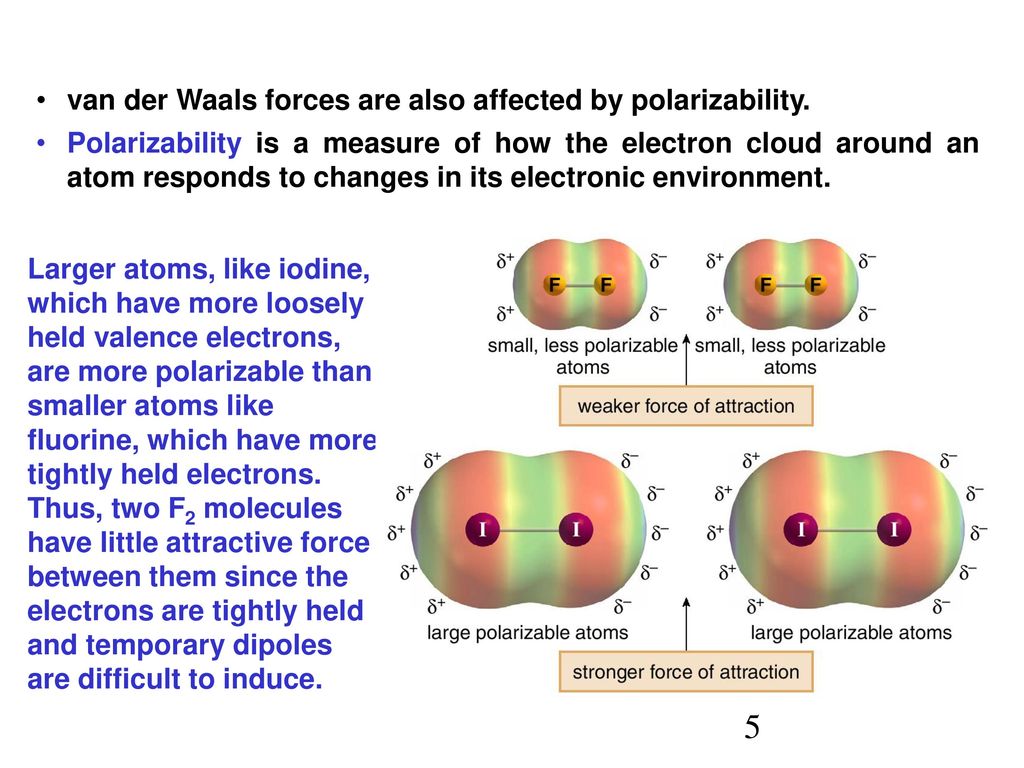
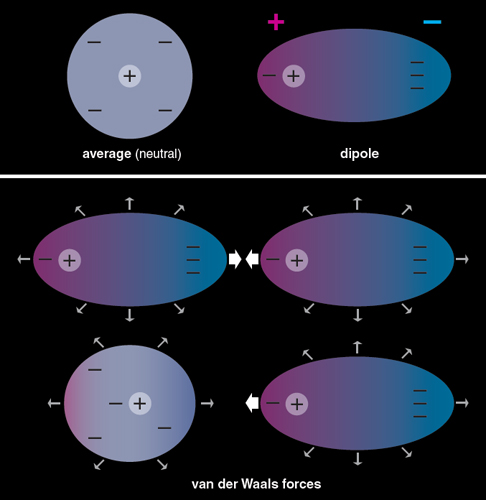
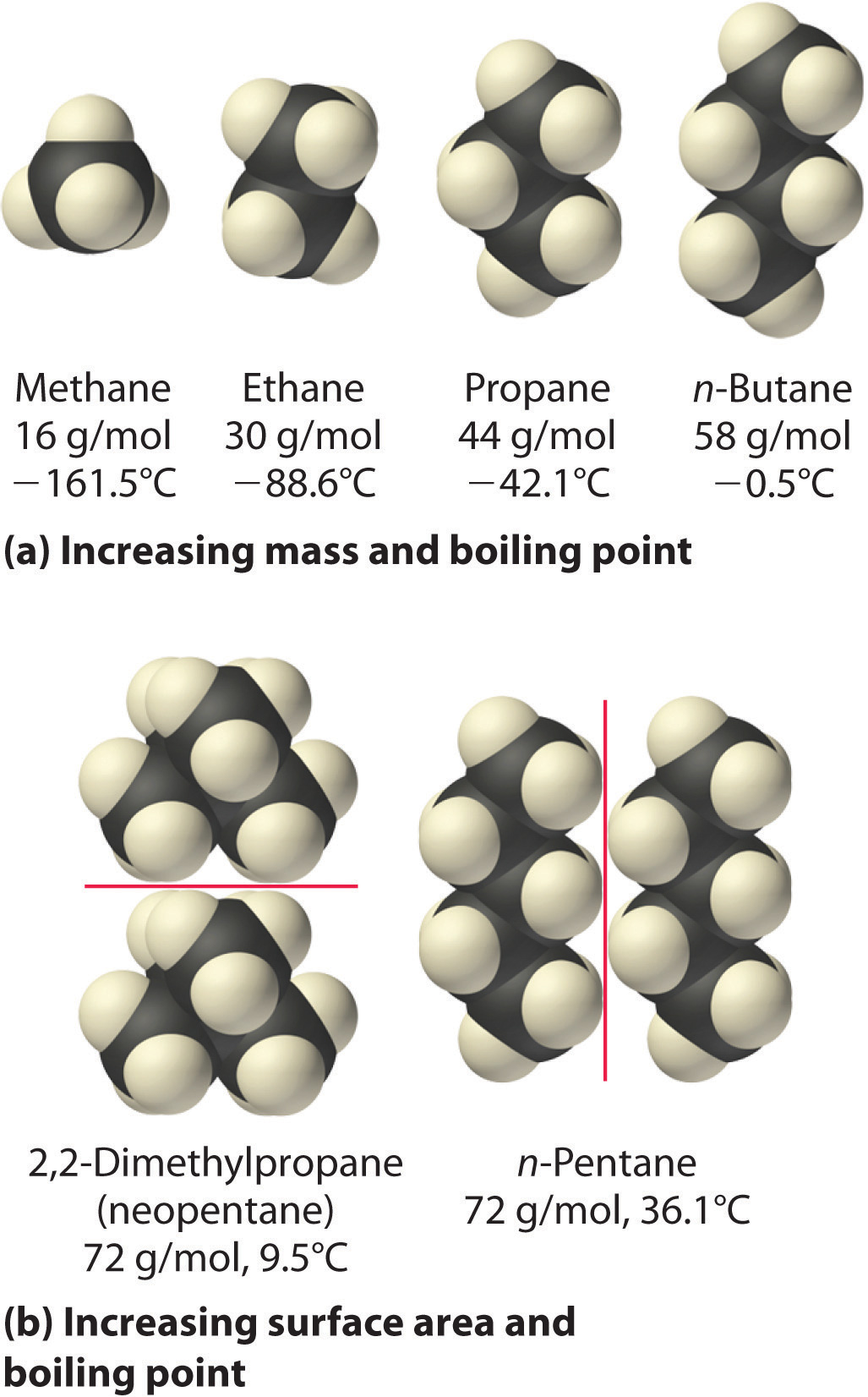
Communication: Non-additivity of van der Waals interactions between nanostructures: The Journal of Chemical Physics: Vol 141, No 14
Effects of van der Waals interactions on the polarizability of atoms, oscillators, and dipolar rotors at long range: The Journal of Chemical Physics: Vol 75, No 6
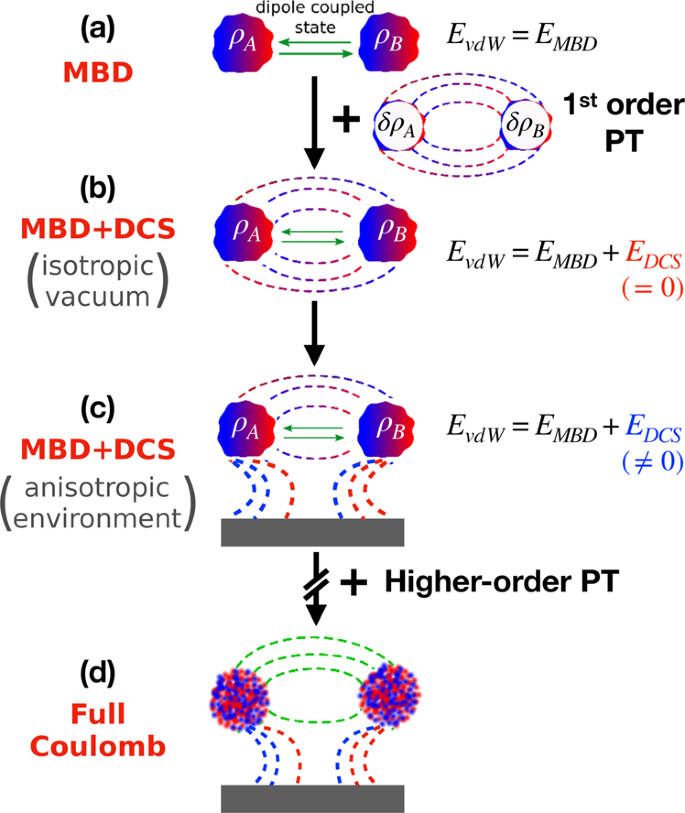
Coulomb interactions between dipolar quantum fluctuations in van der Waals bound molecules and materials | Nature Communications

Image method in the calculation of the van der Waals force between an atom and a conducting surface: American Journal of Physics: Vol 81, No 5
![PDF] Theoretical investigation of the potential energy, dipole moment and polarizability surfaces of the CH4 - N2 and C2H4 - C2H4 van der Waals complexes | Semantic Scholar PDF] Theoretical investigation of the potential energy, dipole moment and polarizability surfaces of the CH4 - N2 and C2H4 - C2H4 van der Waals complexes | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/606eadef63b76ddc96930bb6b88cf6a27beb68dd/21-Figure1.1-1.png)
PDF] Theoretical investigation of the potential energy, dipole moment and polarizability surfaces of the CH4 - N2 and C2H4 - C2H4 van der Waals complexes | Semantic Scholar